Post by lordrye on Dec 18, 2007 5:09:07 GMT -5
Recently I've read physics involving the energy levels on subatomic levels and how those are upheld and affected by energies.
I've pondered a bit about the possibilities of applying physics to my endeavors in the world of psi.
[you]Facts[/you]
Let us look into this a bit further. Let's take the hydrogen atom as an example. The hydrogen atom consists of 1 proton(positively charged particle) and 1 neutron(neutrally charged particle) in the core with 1 electron(negatively charged particle) orbiting it.
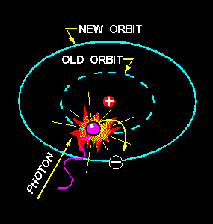
Now, let us visualize the proton, neutron and electron, in their states. Let's say a photon crashes with the hydrogen's electron and some energy is transferred. If enough energy is transferred the electron will jump to a higher energy level. Electron energy levels often start at nil from the core and increase the farther you go.
The Electron jumps up one energy level. Somehow the electron, let us say it feels "unsafe"(I know, very unscientific), can't stay and jumps back. On the route to where the electron jumps back it emits all it's excess energy in the shape of a photon.
Electrons in the lower energy levels will always jump back when they've reached a higher energy level, and energy will always be emitted when it jumps back; often in the shape of a photon.
If you add enough energy to an atom the atom emits a photon (for most stable atoms(not radioactive)) which we see as light. Depending on the amount of energy we add to the atom different colors will be emitted.
[you]Hypothesis[/you]
By adding energy to the atoms around us, through psi, we are able to manufacture photons, so to speak. If enough atoms emit photons it can be visible to the naked eye.
Exciting(adding energy) that many atoms would probably take it's share of psi.
The fact part was for the layman to better visualize the process.
Edit: Corrected grammatical as well as linguistic errors.
I've pondered a bit about the possibilities of applying physics to my endeavors in the world of psi.
[you]Facts[/you]
Let us look into this a bit further. Let's take the hydrogen atom as an example. The hydrogen atom consists of 1 proton(positively charged particle) and 1 neutron(neutrally charged particle) in the core with 1 electron(negatively charged particle) orbiting it.

Now, let us visualize the proton, neutron and electron, in their states. Let's say a photon crashes with the hydrogen's electron and some energy is transferred. If enough energy is transferred the electron will jump to a higher energy level. Electron energy levels often start at nil from the core and increase the farther you go.
The Electron jumps up one energy level. Somehow the electron, let us say it feels "unsafe"(I know, very unscientific), can't stay and jumps back. On the route to where the electron jumps back it emits all it's excess energy in the shape of a photon.
Electrons in the lower energy levels will always jump back when they've reached a higher energy level, and energy will always be emitted when it jumps back; often in the shape of a photon.
If you add enough energy to an atom the atom emits a photon (for most stable atoms(not radioactive)) which we see as light. Depending on the amount of energy we add to the atom different colors will be emitted.
[you]Hypothesis[/you]
By adding energy to the atoms around us, through psi, we are able to manufacture photons, so to speak. If enough atoms emit photons it can be visible to the naked eye.
Exciting(adding energy) that many atoms would probably take it's share of psi.
The fact part was for the layman to better visualize the process.
Edit: Corrected grammatical as well as linguistic errors.

